
There are currently no adjuvant therapies recommended
after nephrectomy for patients with renal cell carcinoma
(RCC)
[1]. Drugs that target the vascular endothelial growth
factor receptor (VEGF-R) such as sunitinib and sorafenib are
effective in disease control in the metastatic setting;
however, they rarely completely eradicate the disease
[2] .A number of trials have investigated whether adjuvant
VEGF-targeted therapy can improve outcomes. To date,
results have been reported for two studies
[3,4]. The first
was a randomised phase 3 study (ASSURE) involving
1943 patients with completely resected RCC (pT1b–4 and
any N grade; Supplementary Table 1)
[4]. Patients were
randomised 1:1:1 to sunitinib, sorafenib, or placebo. The
disease-free survival (DFS) and overall survival (OS) results
for sunitinib were hazard ratio (HR) 1.02 (97.5% confidence
interval [CI] 0.85–1.23) and HR 1.17 (97.5% CI 0.90–1.52),
respectively.
In the ASSURE study, 62% of patients in the sunitinib arm
developed grade 3 or 4 toxicity and 34–44% withdrew
(depending on the dosing cohort; Supplementary Table 1).
The sunitinib and sorafenib doses were modified during the
trial to address toxicity concerns. This did not appear to
affect efficacy in an exploratory subset analysis. There was
no survival advantage for either drug, with not even a trend
towards benefit in the treatment arm.
The S-TRAC trial was the second double-blind, placebo-
controlled, randomised phase 3 trial for which results were
reported
[3] .The results contradict ASSURE for DFS. The
S-TRAC study included 615 patients in a 1:1 randomisation.
The HR was 0.76 (95% CI 0.59–0.98;
p
= 0.03) for DFS, and OS
was immature. Grade 3/4 toxicity in the study was 60.5% for
patients receiving sunitinib, which translated into signifi-
cant differences in quality of life in terms of loss of appetite
and diarrhoea. The study was smaller than ASSURE and had
shorter follow-up. While the majority of patients over-
lapped, S-TRAC consisted of a population at slightly higher
risk. Furthermore, there was no central radiologic review in
ASSURE. This point is important because the DFS HRs, the
primary endpoint of the trials, were different (central
review for S-TRAC and investigator review for ASSURE,
Supplementary Table 1). It is noteworthy that the investi-
gator assessment for DFS in S-TRAC did not reach statistical
significance (HR 0.81, 95% CI 0.64–1.02;
p
= 0.08), as was the
case with ASSURE.
These data prompt a number of questions that need to be
addressed before we can consider changing the standard of
care. First, is DFS a good surrogate endpoint in this setting?
OS takes longer to achieve, and DFS has at times translated
into an OS advantage in the adjuvant setting in other
diseases
[5–7]. In breast cancer trials, DFS HRs
>
0.76
without OS benefit have resulted in regulatory approval and
adoption as a standard of care
[6]. Therefore, DFS is a
reasonable endpoint and a precedent exists, but DFS needs
to translate into OS in the long run
[7]. The assumption that
this OS signal is inevitable with S-TRAC is questionable
owing to the preliminary OS results in ASSURE and S-TRAC
(HR 1.01, 95% CI 0.72–1.44;
p
= 0.94 for S-TRAC; HR 1.17,
97.5% CI 0.90–1.52;
p
= 0.1762 for ASSURE, Supplementary
Table 1). There are also uncertainties for the current
treatment algorithm for metastatic RCC. Treatment for
first-line metastatic renal cancer is VEGF-targeted therapy,
as is being tested in the adjuvant setting. Cross-resistance
between VEGF-targeted therapies occurs, and it is possible
that patients who relapse early on sunitinib will do less well
with subsequent VEGF-targeted therapy
[8] .This has been
described as ‘‘the law of diminishing returns’’ in VEGF-
resistant metastatic RCC
[9]. Currently there is no indication
of even a trend towards a survival advantage in either study,
although the data are immature. To achieve positive results
after initially overwhelming negative OS results to date,
much later analysis after longer follow-up will be required.
The results would need to show the Kaplan-Meier OS curves
parting significantly after a number of years of follow-up in
a nonproportional HR manner. It seems uncertain that this
will occur from the data available to us.
In reality, the definition of early metastatic disease is a
grey area. The presence of metastasis is determined via
cross-sectional imaging, which is only able to identify
sizeable cancer deposits from a molecular perspective.
Therefore, large numbers of patients with high-risk disease
probably have early, as yet undetected, metastatic disease.
Intervention with sunitinib in these patients will essentially
be treating early metastatic disease. Stabilisation with
sunitinib, especially at the beginning of the intervention
period, would therefore be expected. Perhaps a more
pertinent question is why this was not seen in ASSURE.
Nevertheless, the data available to us do not currently
support the hypothesis that VEGF-targeted therapy can
prevent relapse and extend survival.
What we can confidently say is that there is an issue
around tolerability, which was consistent in both studies. A
year of therapy clearly comes with significant toxicity that
can affect quality of life.
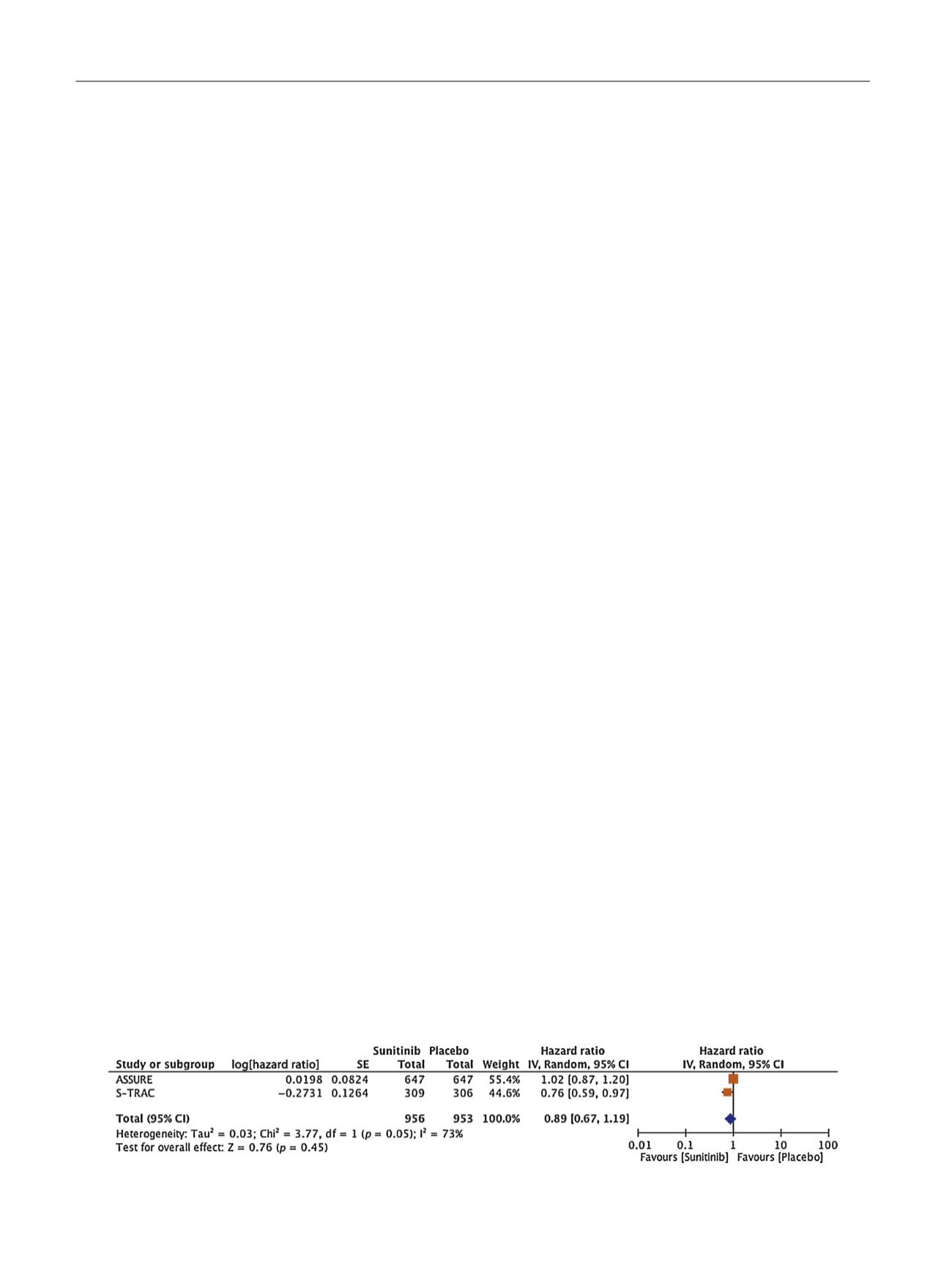
Where multiple studies exist, meta-analysis is a power-
ful tool. Again, for breast cancer and other tumour types,
meta-analysis is the driver that influences guidelines, rather
than a single study
[5,7,10]. Results from a meta-analysis of
ASSURE and STRAC trials show statistically nonsignificant
results for DFS owing to the sheer size of ASSURE
( Fig. 1;
combined HR 0.89, 95% CI 0.67–1.19). OS is immature, but
[(Fig._1)TD$FIG]
Fig. 1 – Meta-analysis of disease-free survival (DFS) for S-TRAC and ASSURE. CI = confidence interval; SE = standard error; df = degrees of freedom.
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 7 1 9 – 7 2 2
720
















