
negative at this stage. This meta-analysis has shortcomings
such as subtle differences in patient populations and
methods of assessment, but it is an important piece of
the jigsaw.
In conclusion, it is preferable to look at the adjuvant
studies together rather than in isolation. The outcomes of
the placebo arms of the two trials are similar, which is
reassuring from a trial conduct perspective. The results for
DFS are contradictory and the reason is not clear. Despite
imbalances in risk assessment, inclusion of non–clear cell
RCC, and starting dosages, these studies have much more in
commonwith one another than differences (Supplementary
Table 1). The DFS at 5 yr in the placebo arm of both studies is
almost identical. The pathological T3–4 subgroup in ASSURE
matching the patients included in S-TRAC is even numeri-
cally stronger and did not reveal a benefit for DFS (HR 1.04,
95% CI 0.83–1.31) or OS in exploratory subgroup analysis
[4]. Without a consistent trend towards a DFS signal, it is not
possible to confidently say that there is likely to be a
survival benefit. The one area for which there is clarity is
toxicity, which is an important consideration. Consider-
ations for the future include the outcomes of other trials in
the area and long-term OS data.
The European Association of Urology Renal
[1_TD$DIFF]
Cell Cancer
Guidelines Panel, which includes patient representatives
and clinicians, considered a number of different scenarios to
determine what would be required from S-TRAC to change
practice. The decision on whether to change practice was
taken in the context of the data available from ASSURE, and
the assumption that the level of toxicity with sunitinib
would be in line with those seen previously. Results showed
that only 1/15 (6%) of the panel would change their
standard of care when considering the DFS and OS closest to
S-TRAC (DFS: HR 0.75,
p
<
[2_TD$DIFF]
0.05; OS: HR 1.0,
p
>
0.05).
Standard practice would only be significantly influenced by
a significant survival benefit
( Fig. 2). In addition, kidney
cancer patients from the International Kidney Cancer
Coalition (IKCC) participated in a questionnaire about the
implications for STRAC. The results lacked clarity (Supple-
mentary Fig. 1,
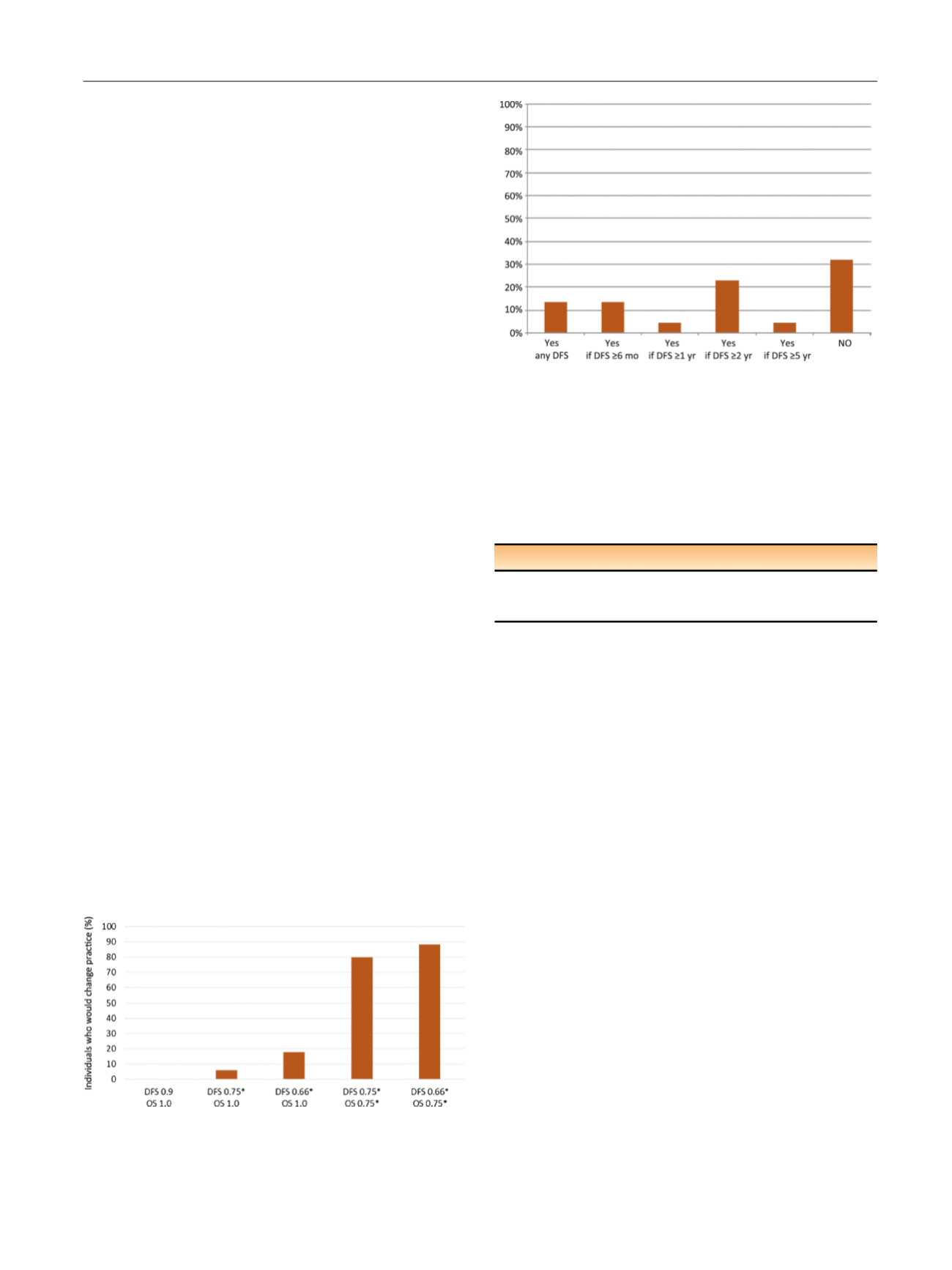
Fig. 3 ). Twenty-two patient representatives
from the IKCC network were asked what degree of PFS
advantage would be needed to justify taking sunitinib for
1 yr. Approximately one-third of patients favoured not
taking sunitinib when faced with the S-TRAC results
( Fig. 3).
Finally, the panel summarised the current evidence
(Supplementary Table 2) and judged the strength of the
recommendation (Supplementary Table 3). This resulted in
the following recommendation, towhich 80% of the 15 panel
members entitled to vote strongly agreed via anonymous
voting (Supplementary Fig. 2). The final recommendation
against adjuvant sunitinib reflects the poor benefit-to-harm
ratio and the current absence of evidence of an OS benefit
( Table 1).
The strength of the recommendation in
Table 1is weak
because the panel took into account that some patients
would favour having this option despite the toxicity, the
unproven OS benefit, and the overall weak quality of the
evidence.
Author contributions:
Axel Bex had full access to all the data in the study
and takes responsibility for the integrity of the data and the accuracy of
the data analysis.
Study concept and design:
Bex, Powles.
Acquisition of data:
Bex, Powles.
Analysis and interpretation of data:
Bex, Albiges, Ljungberg, Bensalah,
Dabestani, Giles, Hofmann, Hora, Kuczyk, Lam, Marconi, Merseburger,
Staehler, Volpe, Powles.
Drafting of the manuscript:
Bex.
Critical revision of the manuscript for important intellectual content:
Bex,
Albiges, Ljungberg, Bensalah, Dabestani, Giles, Hofmann, Hora, Kuczyk,
Lam, Marconi, Merseburger, Staehler, Volpe, Powles.
[(Fig._2)TD$FIG]
Fig. 2 – Members were asked which results from S-TRAC would
change their standard practice in the context of the data available
in ASSURE and toxicity profiles consistent with those seen for sunitinib.
* Statistically significant. DFS = disease-free survival; OS = overall
survival.
[(Fig._3)TD$FIG]
Fig. 3 – Patient representatives from the IKCC network (
n
= 22) were
asked: ‘‘After surgery for kidney cancer, if your doctor told that you are
at high risk of recurrence (spread), would you consider taking sunitinib
(Sutent) for one year in the hope you could delay the onset of
recurrence even if your overall survival was not improved?’’ Patient
representatives were patients with nonmetastatic disease who had
previously undergone surgery).
Table 1 – Recommendation of the European Association of Urology
Renal
[1_TD$DIFF]
Cell Cancer Guidelines Panel
Recommendation
Strength
Adjuvant sunitinib following surgically resected
high-risk clear-cell renal cell carcinoma is
not recommended
Weak
#
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 7 1 9 – 7 2 2
721
















