
patients, as previously reported
[12]. Not surprisingly,
however,
AR-V7
mRNA levels were much higher in the AR-
driven than in non–AR-driven CRPC metastases, while no
differences in
AKR1C3
mRNA levels were seen (data not
shown and Supplementary Table 2). In addition to AR-
targeted therapies, we hypothesize that patients with AR-
driven CRPC metastases might benefit from therapies
targeting cholesterol biosynthesis,
b
-oxidation, and
polyamine synthesis, pathways that were particularly
upregulated in this subgroup. This is in line with previous
findings by our group of high cholesterol levels and
b
-
oxidation in CRPC bone metastases
[13,14]. Furthermore,
we hypothesize that patients with non–AR-driven CRPC
metastases with preserved MHC class I expression will be
resistant to all forms of anti-AR therapy, but might instead
be susceptible to cancer immunotherapy.
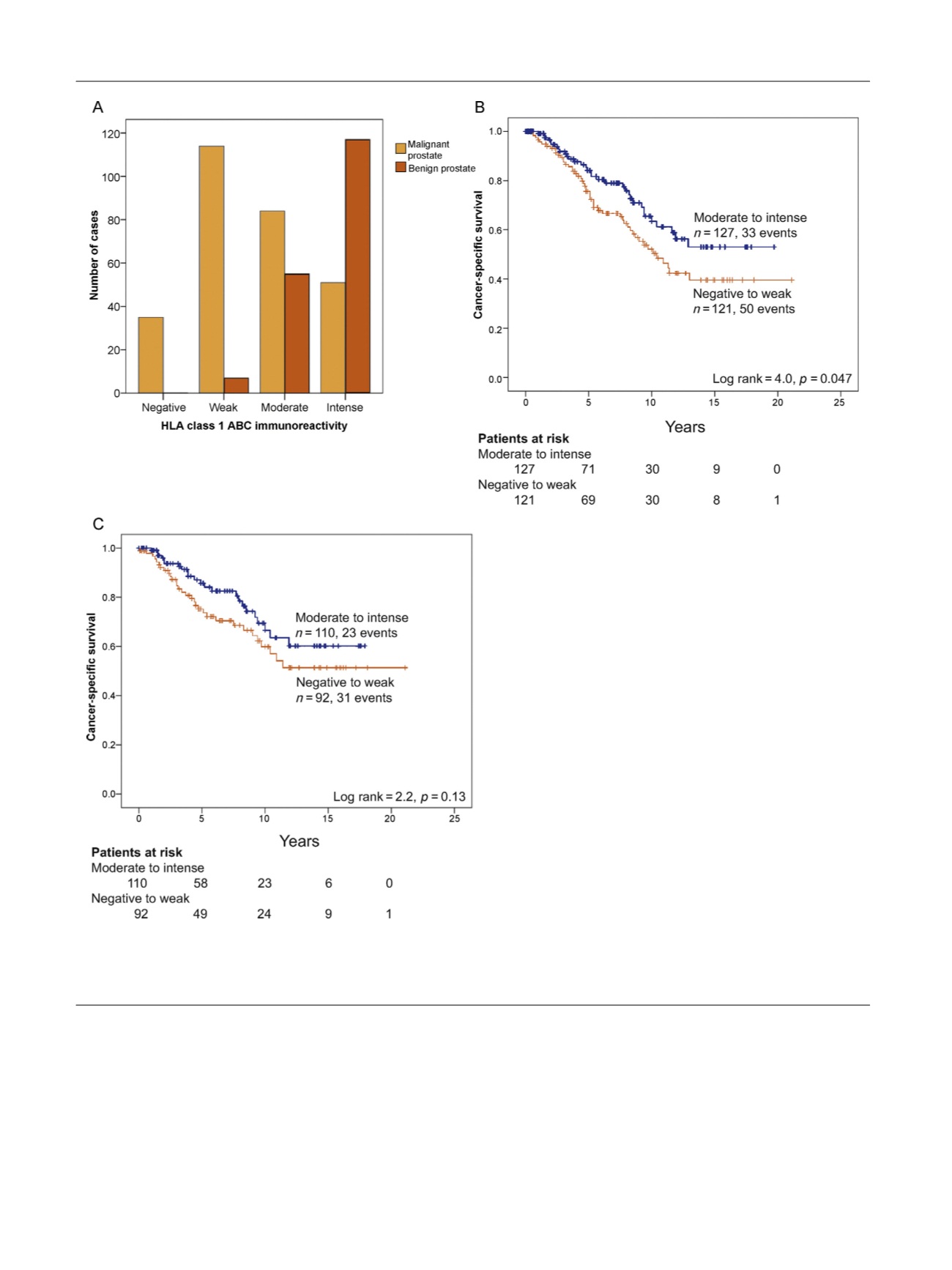
[(Fig._5)TD$FIG]
Fig. 5 – Tumor immunoreactivity for HLA class I ABC in a historical cohort of patients diagnosed via transurethral section of the prostate showing (A)
reduced staining intensity in malignant (
n
= 284) compared to adjacent nonmalignant (
n
= 179) epithelium (
p
< 0.001) and shorter cancer-specific
survival in patients with lower HLA class I tumor cell immunoreactivity (negative to weak) compared to
[1_TD$DIFF]
patients with moderate to intense
immunoreactivity in
[9_TD$DIFF]
(B) patients without metastases at diagnosis (
p
= 0.047,
n
= 248), and (C) patients treated with watchful waiting (
p
= 0.13,
n
= 202).
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 7 7 6 – 7 8 7
784
















