
Thus, we focused on long-term clinical outcomes defined
identically between groups. Secondly, it is possible that the
upfront usage of ADT in the EBRT + BT cohort, which was not
used in the RP cohort, could explain the differences in DMFS.
However, upfront use of ADT is not the standard of care for
patients undergoing RP. Indeed, while multiple studies have
shown that upfront ADT with RT improves OS, upfront ADT
with RP has never demonstrated clinical benefit except in
the case of patients with pN+
[25,26]. Eighteen of the 28 pN+
patients received adjuvant ADT due to frequent refusal,
accounting for 10.5% of the RP cohort. A higher percentage
of EBRT + BT patients did not receive ADT (13.8%), which is
also substandard care. Even when pN+ patients are
excluded, DMFS remains improved in the EBRT + BT cohort.
Further, emerging data suggests that neoadjuvant ADT acts
as a radiosensitizer, while adjuvant ADT blocks RT-induced
androgen receptor signaling
[27,28]. Thus, the effects of
ADT in the EBRT + BT patients may not readily be
extrapolated to patients undergoing RP.
Our results cannot be ascribed to inferior outcomes in
the RP cohort. The largest prior surgical series included
259 patients with bGS 9–10 disease
[20] .Our surgical
cohort had more patients with positive margins (40.6% vs
36.4%) and seminal vesicle invasion without pN+ (36.5%
vs 22.4%), but a similar percentage with pN+ disease (16.5%
vs 17.4%). Our 5-yr and 10-yr CSS rates of 91.7% and
78.5% compare favorably with that study’s reported rates of
92% and 60.7%, respectively. Additionally, a recent multi-
institutional series including 1051 RP reported 5-yr and
8-yr BCRFS rates of 25% and 15%, comparable to our 5-yr
and 10-yr rates of 26.4% and 16.2%
[17]. Our results also
compare favorably with previously reported outcomes of
patients with bGS 9-10 treated with either RP or EBRT
[16,19]. Tsao et al.
[16]recently reported 5-yr BCRFS and
DMFS rates of approximately 40% and 60%, respectively, in a
cohort of 363 patients treated with RP or EBRT for bGS 9–10
CaP, compared with rates of 81.9% and 58.6%, respectively,
in the entire population for the current study.
Our finding that EBRT + BT provides improved systemic
control over both EBRT and RP in this setting is novel, and
suggests that optimal local control (offered by extreme dose-
escalation) and an upfront method of systemic control
(offered by a frequent use of ADT in this cohort) may
represent the best upfront treatment strategy for these
patients who are at high risk of harboring micrometastatic
disease at presentation. A link between local control and
systemic control has been previously suggested
[9,10,29–32],
and the results of a randomized trial have suggested a DMFS
benefit to dose-escalated RT
[10]. We chose EBRT + BT as a
model for extremely dose-escalated RT given the availability
of long-term clinical outcomes. Preliminary results of
the ASCENDE-RT trial, where randomized patients with
intermediate- or high-risk CaPwere given EBRT alone or EBRT
with an LDR-BT boost to demonstrate a progression-free
survival benefit
[32]. Because themediandurationof ADTwas
actually lower in the EBRT + BT cohort, the improvedsystemic
control between the EBRT and BT cohorts is likely attributable
to dose-escalation. While the benefits of ADT may not be
immediately extrapolated froma RT setting to a RP setting, as
discussed above, the difference in systemic control between
EBRT + BT and RP may conceivably be related to a systemic
effect of even short duration ADT on micrometastatic disease
in the majority of patients in the EBRT + BT cohort. In that
case, upfront use of hormonal- or chemotherapy-based
systemic agents with RP may provide better outcomes.
Nonetheless, it must be emphasized that despite a systemic
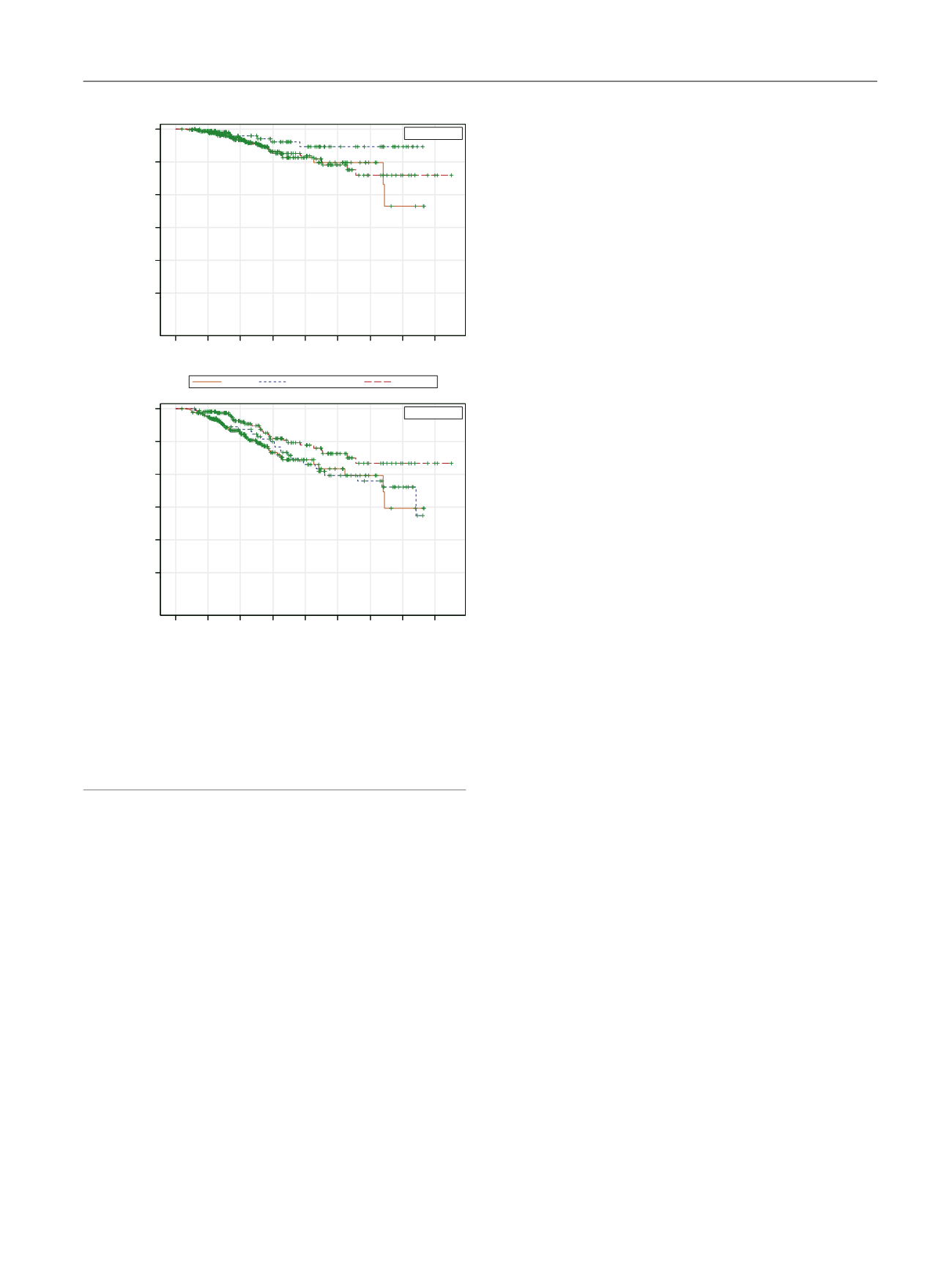
control benefit, no differences were found in CSS or OS. This
may be due to limited power with a relatively smaller
EBRT + BT cohort, the utilization of effective systemic salvage
modalities at the time of metastatic disease, and/or a longer
natural history for death after metastatic disease than
originally assumed.
This work has several limitations. Primarily, because this
was a retrospective analysis, the treatments within cohorts
are not homogeneous; for example, ADT duration, EBRT
dose, postoperative EBRT strategies were heterogeneous,
and follow-up protocols were not standardized. Further, to
maximize power, we pooled data from several institutions,
likely compounding this issue. Therefore, our results are
primarily hypothesis-generating and will need prospective
[(Fig._2)TD$FIG]
229 208 123 55 28 18 8 3 0
81 74 55 44 33 20 16 7 0
170 161 100 72 51 31 16 8 2
0 2 4 6 8 10 12 14 16
Time (yr)
0.0
0.2
0.4
0.6
0.8
1.0
(A)
(B)
Cancer-specific survival probability
EBRT
EBRT + brachy
Surgery
Surgery
EBRT
+ Censored
EBRT+BT
EBRT
RP
230 207 124 55 28 18 8 3 0
87 80 61 49 35 21 17 8 0
170 161 100 72 51 31 16 8 2
0 2 4 6 8 10 12 14 16
Time (yr)
0.0
0.2
0.4
0.6
0.8
1.0
Overall survival probability
EBRT
EBRT + brachy
Surgery
+ Censored
EBRT
EBRT+BT
RP
EBRT + brachy
Fig. 2 – (A) Kaplan-Meier curves for cancer-specific survival. (B) Kaplan-
Meier curves for overall survival. The curves have not been adjusted for
age, Gleason score, clinical stage, or prostate-specific antigen. Following
multivariate regression adjusted for these factors, all patients had
statistically similar 5-yr and 10-yr cancer-specific survival and overall
survival rates.
brachy = brachytherapy; EBRT = external beam radiotherapy; RP = radical
prostatectomy.
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 7 6 6 – 7 7 3
771
















