
better in RCT-eligible versus all RW patients (
p
<
0.05,
Supplementary Fig. 4). However, during the 1st yr, Team 1,
Team 3, and the Halabi model performed significantly
worse in RCT-eligible patients (
p
<
0.05). Although overall
OS was not different between the RCT-eligible and none-
ligible RW patients (
p
= 0.70; Supplementary Fig. 5), the
RCT-eligible patients had decreased mortality during the
1st follow-up yr (
p
<
0.01). This supports clinical validity of
RCT eligibility criteria in assessing patient’s likelihood of
benefitting from docetaxel.
Taken together, our results confirm the importance of
supplementing RCTs with RW data and the utility of current
gold-standard prognostic models in RW mCRPC patients.
Conflicts of interest:
The authors have nothing to disclose.
Acknowledgments:
Seyednasrollah received a grant from the Doctoral
Program in Mathematics and Computer Sciences at the University of
Turku. Rautakorpi received a grant from the Cancer Society of Finland.
Elo received grants from the European Research Council, European
Union’s Horizon 2020 Research and Innovation Program, Academy of
[(Fig._1)TD$FIG]
C
B
RealWorld Cohort (N=289)
Clinical Trial Cohort (N=313)
Time-dependent AUC
Time (months)
Time-dependent AUC
Time (months)
0.75
0.70
0.65
0.60
0.55
0.90
0.85
0.80
0.75
0.70
0.65
0.60
0.55
0.90
0.85
0.80
16 14 12 10 8 6
30 28 26 24 22 20 18
Team 1
Team 2
Team 3
Halabi
16 14 12 10 8 6
30 28 26 24 22 20 18
Team 1
Team 2
Team 3
Halabi
Principal Component 1
Principal Component 2
−
2
0
2
6
4
−
0
2
4
2
RW cohort
ENTHUSE 33
ASCENT2
MAINSAIL
VENICE
A
Halabi model clinical features
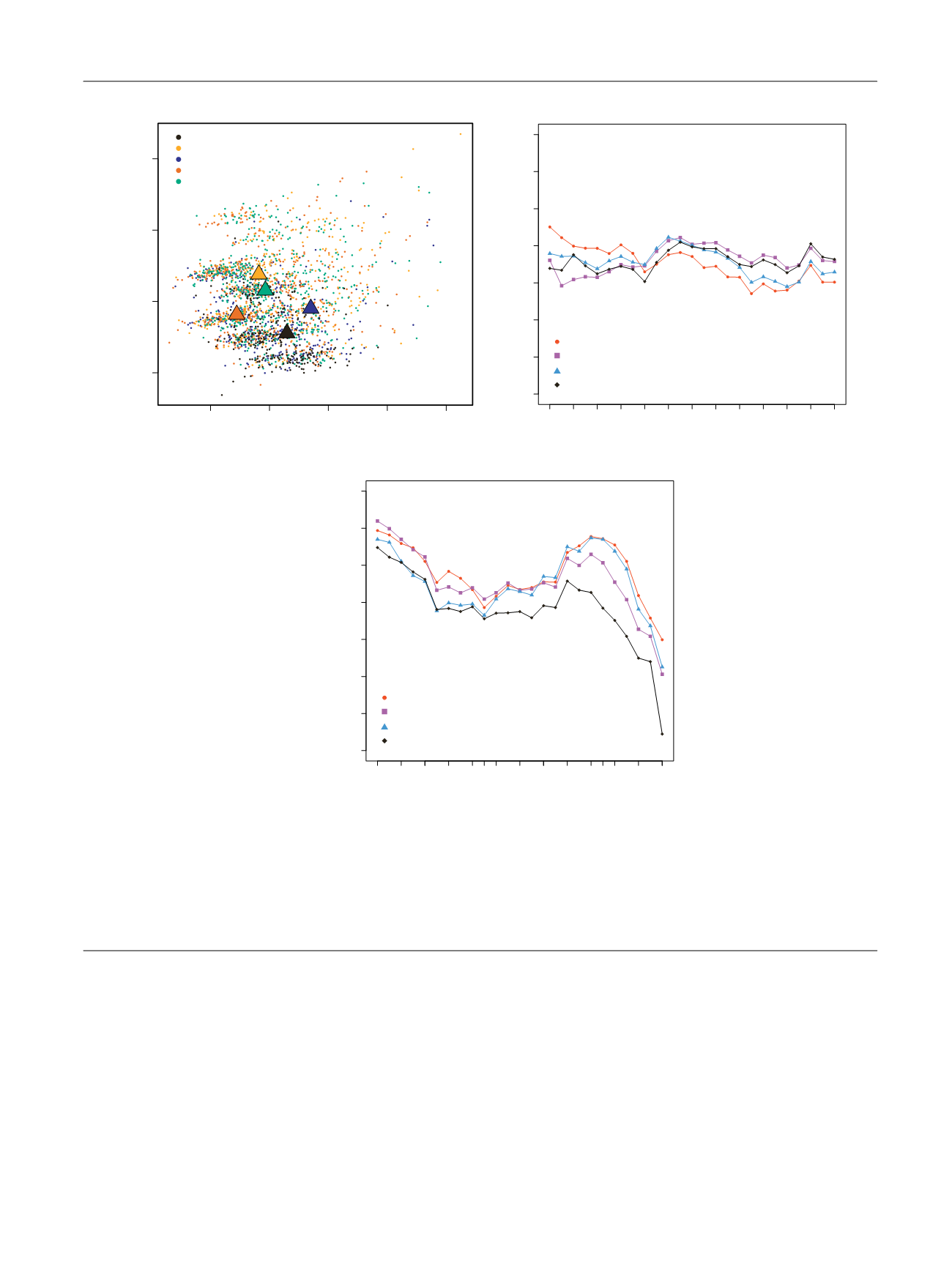
Fig. 1 – (A) Principal component analysis of the four randomized clinical trial datasets ASCENT2 (
n
= 476), MAINSAIL (
n
= 526), VENICE (
n
= 598),
ENTHUSE 33 (
n
= 470), and the real-world (RW) cohort (
n
= 289). Principal component analysis was performed using the variables from the Halabi
model, including analgesic use, metastasis site (defined as lymph node only, bone metastases with no visceral involvement, or any visceral
metastases), Eastern Cooperative Oncology Group performance status, lactate dehydrogenase, albumin, hemoglobin, alkaline phosphatase, and
prostate-specific antigen. (B) Performance of the selected four models in the RW cohort (Turku University Hospital, Finland,
n
= 289) and (C) the
randomized clinical trial validation cohort (ENTHUSE 33,
n
= 313). The ENTHUSE trial included 470 patients of which 313 patients were used as the
final method evaluation in the Dialogue for Reverse Engineering Assessments and Methods Challenge. The performance of the models in predicting
the overall survival at different time points was measured using the time-dependent area under the curve (AUC) from 6 mo to 30 mo with 1-mo
intervals.
E U R O P E A N U R O L O G Y 7 1 ( 2 0 1 7 ) 8 3 7 – 8 4 3
839
















